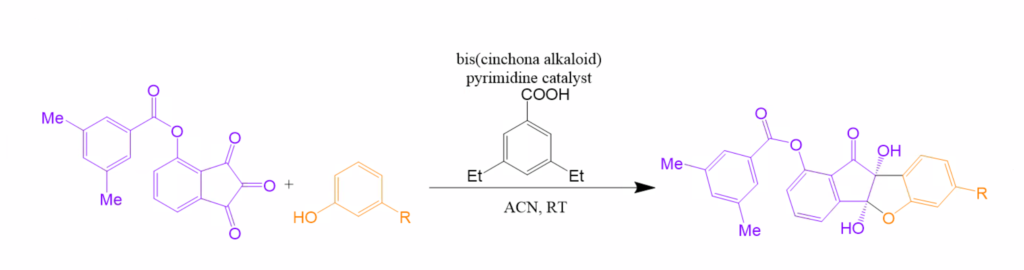
Abstract: There is a demonstrated need for drugs targeting diseases, such as the flu, polio, and hepatitis A, caused by the Picornaviridae family. It has been established that natural and synthetic benzofuran derivatives have effective antimicrobial, antitumor, and analgesic activities. Namely, benzo[b]indeno[2,1-d]furanone, a benzofuran, exhibits effective inhibition against picornavirus at nanomolar concentrations and also has low cytotoxicity towards host cells. A report from Kim et al. constructed a novel picornavirus inhibitor that is a small, stereochemical complex benzofuroid. To make this biologically active compound, we have developed a pathway using a catalytic asymmetric addition Interrupted Feist- Bѐnary (IFB) reaction that would selectively set the stereochemistry of the final target. IFB reactions usually create racemic mixtures, however, only one enantiomer is functional. The modified catalytic IFB reaction involves enantioselective reactions between 1,2,3-indanetrones and substituted phenols catalyzed by bis(cinchona-alkaloid)pyrimidine catalysts. This stoichiometric acid addition yields chiral cyclic hemiacetals that we should be able to convert into the benzofuranoid antiviral discussed above. In the newly developed modified catalytic IFB reaction, the final chiral cyclic hemiacetal has three possible configurations, producing six isomers in our final product. Exposure to acids causes hemiacetal products to rearrange into different conformations. Our goal is to isolate each configuration, and then convert them into the antiviral compound by reducing out one oxygen and converting the hemiacetal into an amine.
Video:
Live Poster Session:
Thursday, July 29th 1:15-2:30pm EDT

